Elektroauto wie Benzineraufladen - STROMTANKSTELLE
Da das Elektroauto mit Strom statt mit Benzin fährt, blieb einem bisher nur das aufladen des Akku oder die Batterie auszutauschen. Das Aufladen der Batterie für ein Elektroauto dauert an einer normalen Steckdose mitunter bis zu acht Stunden. Nun haben Wissenschaftler des MIT einen Akku entwickelt, dessen Elektrolyt aus einer zähflüssigen Masse besteht. Weil das Elektrolyt flüssig ist, lässt sich der Stromspeicher auch von außen wie ein Fahrzeug mit einem Verbrennungsmotor auftanken oder wahlweise wie ein normaler Akku aufladen.
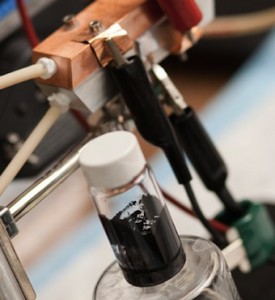
- Im Glas sieht man das "Cambridge crude". Bildquelle: MIT
Die Forscher des MIT nennen das flüssige Elektrolyt auf Anspielung auf das Crude-Oil der Ölwirtschaft "Cambridge crude". Das Mittel funktioniert in einer Flussbatterie, bei der die Ladungsträger nicht, wie beispielsweise bei Trockenbatterien, in fester Form vorliegen.
Der Vorteil ist, das der Akku durch den Austausch des flüssigen Elektrolyts schnell wieder mit Strom aufgeladen werden kann, die Forscher sehen dabei ausdrücklich auch das Elektroauto als Anwendungsgebiet. Hier könnte das Elektrolyt wie Benzin an einer Tankstelle in das Elektrofahrzeug gefüllt werden, das nicht aufgeladene Elektrolyt würde in dem Fall aus der Batterie abgesaugt werden müssen.
Man kann den Akku aber auch ganz normal mit Strom aufladen, so kann man zum Beispiel zu Hause sein Elektroauto ganz normal an eine Steckdose anschließen. Der Fahrer hat daher die Wahl, ob er schnelle Energie durch Nachtanken oder langsame durch Aufladen beziehen möchte. Da heute bereits so gut wie alle Fahrzeuge mit flüssigen Energieträgern wie Benzin gefüllt werden, sucht die Wissenschaft und die Industrie schon lange nach einer Alternative zum Öl in flüssiger Form – denn so ließen sich die Tankstellen einfacher umrüsten und die Ladeinfrastruktur wäre bereits vorhanden.
Die Materialien des auch "semi-solid flow cell" (SSFC) genannten Akkus des MIT basieren auf Lithium, die Energiedichte soll laut des MIT sogar höher sein als bei herkömmlichen Lithiumionen-Zellen. In der Endentwicklung rechnen die Forscher mit der Hälfte der Größe einer bisherigen Zelle. Die Forscher haben ihre Ergebnisse in der Fachzeitschrift Advanced Energy Materials veröffentlicht.
Der Preis für das "Cambridge crude" ist derzeit noch nicht bekannt, allerdings kann man davon ausgehen, das die Rohölkonzerne sehr bald das Patent für die "flüssige Batterie" kaufen werden, denn welche Konzerne wollen eine Konkurrenz zum Öl entstehen lassen, so lange es noch verhältnismäßig günstig ist?

Battery technology under development at MIT could someday make recharging batteries as quick and easy as a trip to the gas station.
Known as semi-solid flow cells, the new battery design turns the chemistry of traditional lithium-ion batteries into quicksand-like tiny particles. The resultant slime — which researchers jokingly call "Cambridge crude" — has an extremely high energy density and is cheaper to manufacture than the innards of a traditional lithium-ion battery. The researchers claim battery cost and size could be cut in half as a result.
That "fuel" offers another advantage: it can be refilled as well as recharged thanks to the new battery's aqueous-flow structure, in which positive and negative electrodes are solid particles suspended in a liquid electrolyte. Instead of plugging in to recharge, you could simply refill the depleted liquid at a refueling station. Are you reading this, Shai Agassi?
Flow batteries have been around for awhile but have used liquids with low energy density, making them too large for practical use in EVs. The new high energy density fluid makes the batteries lighter than lithium-ion counterparts. That could increase the range of the EVs in which they're installed.
"We're using two proven technologies, and putting them together," said materials science professor W. Craig Carter, who led development of the new battery technology along with professor and A123 co-founder Yet-Ming Chiang, undergraduate Mihai Duduta and graduate student Bryan Ho.
Automotive designers will be glad to learn that there are space-saving advantages to the new architecture, too. While a conventional battery combines storage and discharge functions, these new batteries keep the two functions separate. That makes it possible to separate the storage and discharge components, eliminating the need for automakers to design a space-wasting battery storage "tunnel" that's been a feature of all electric cars to this point.
Development of the technology was partially funded by the Department of Defense. The batteries are licensed to A123 Systems spinoff 24M in Cambridge, Massachusetts
MEET the Vanadium Battery
The vanadium redox (and redox flow) battery is a type of rechargeable flow battery that employs vanadium ions in different oxidation states to store chemical potential energy. The present form (with sulfuric acid electrolytes) was patented by the University of New South Wales in Australia in 1986 [2] An earlier German Patent on a titanium chloride flow battery was registered and granted in July 1954 to Dr. Walter Kango, but most of the development of flow batteries was carried out by NASA researchers in the 1970s. Although the use of vanadium in batteries had been suggested earlier by Pissoort,[3] by NASA researchers and by Pellegri and Spaziante in 1978,[4] the first known successful demonstration and commercial development of the all-vanadium redox flow battery employing vanadium in a solution of sulfuric acid in each half was by Maria Skyllas-Kazacos and co-workers at the University of New South Wales in the 1980s.[5]
There are currently a number of suppliers and developers of these battery systems including Ashlawn Energy in the United States, Renewable Energy Dynamics (RED-T) in Ireland, Cellstrom GmbH in Austria, Cellennium in Thailand, and Prudent Energy in China. The vanadium redox battery (VRB) is the product of over 25 years of research, development, testing and evaluation in Australia, Europe, North America and elsewhere.

The vanadium redox battery exploits the ability of vanadium to exist in solution in four different oxidation states, and uses this property to make a battery that has just one electroactive element instead of two.
The main advantages of the vanadium redox battery are that it can offer almost unlimited capacity simply by using larger and larger storage tanks, it can be left completely discharged for long periods with no ill effects, it can be recharged simply by replacing the electrolyte if no power source is available to charge it, and if the electrolytes are accidentally mixed the battery suffers no permanent damage.
The main disadvantages with vanadium redox technology are a relatively poor energy-to-volume ratio, and the system complexity in comparison with standard storage batteries.
Redox flow 1 MWh
Vanadium redox flow battery is one of the well known example.  The Redox Flow Cell is an electrochemical system which allows energy to be stored in two solutions containing different redox couples with electrochemical potentials sufficiently separated from each other to provide an electromotive force to drive the oxidation-reduction reactions needed to charge and discharge the cell. Unlike conventional batteries, the redox flow cell stores energy in the solutions, so that the capacity of the system is determined by the size of the electrolyte tanks, while the system power is determined by the size of the cell stacks. The redox flow cell is therefore more like a rechargeable fuel cell than a battery.
The Redox Flow Cell is an electrochemical system which allows energy to be stored in two solutions containing different redox couples with electrochemical potentials sufficiently separated from each other to provide an electromotive force to drive the oxidation-reduction reactions needed to charge and discharge the cell. Unlike conventional batteries, the redox flow cell stores energy in the solutions, so that the capacity of the system is determined by the size of the electrolyte tanks, while the system power is determined by the size of the cell stacks. The redox flow cell is therefore more like a rechargeable fuel cell than a battery.
This technology can store a huge energy. It has already been demonstrated in windmill and solar installation, UPS system and load leveling substation as big as the 450 kW /1 MWh from Sumitomo Electric Industries in Japan
http://www.youtube.com/watch?v=dWuxQDRyLGw


 Erdmagnetfeld:
Erdmagnetfeld:

0 Comments:
Kommentar veröffentlichen
<< Home